11/7/22
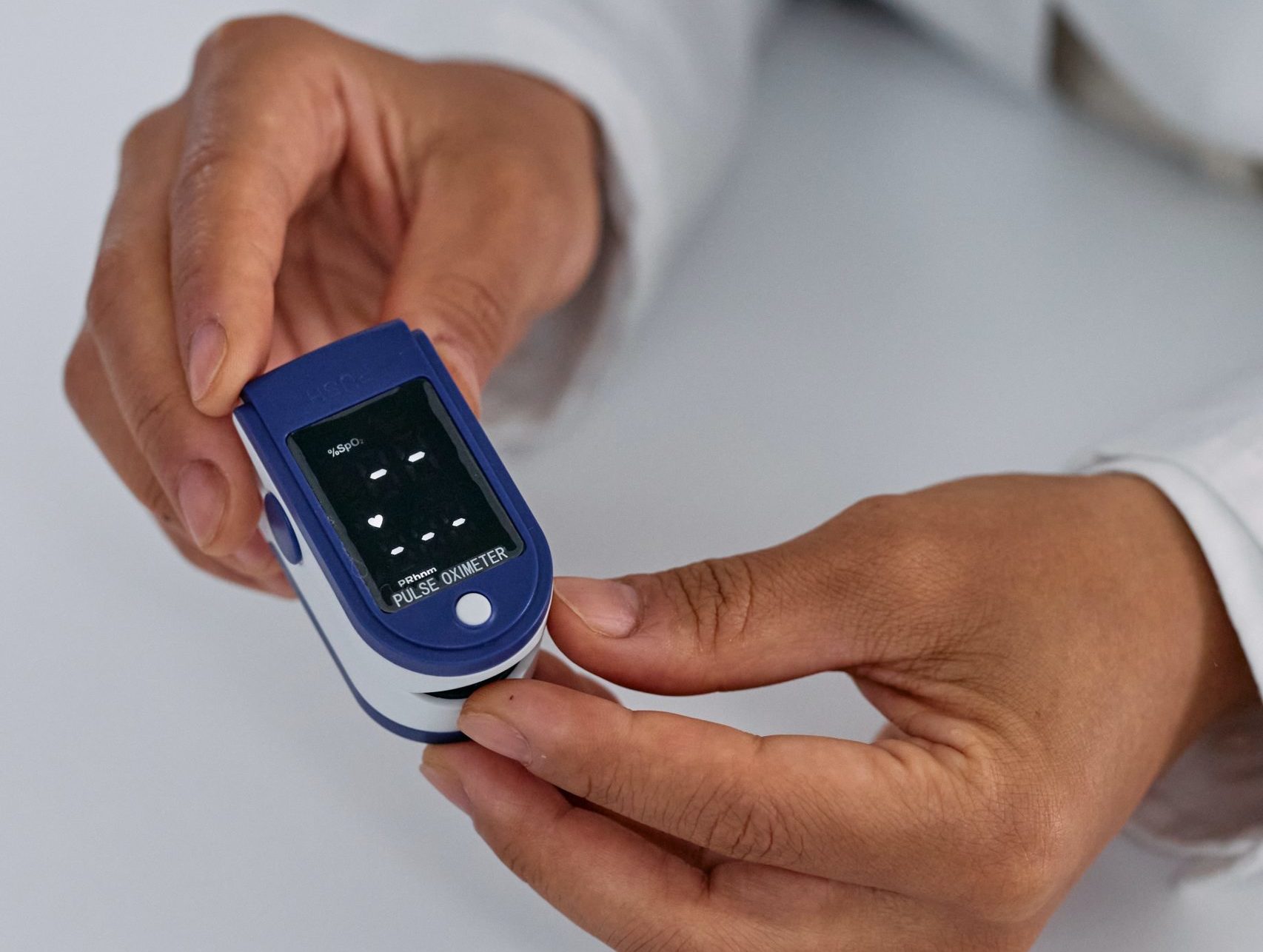
This week, an expert panel of the Food and Drug Administration (FDA) met to provide advice and recommendations to the FDA on regulatory issues, one of which was the accuracy of pulse oximetry among patients with darker skin pigmentation. Pulse oximeters are the ubiquitous medical devices that are the standard for non-invasively assessing a patient’s oxygen levels (also known as oxygen saturation). In this method of measurement, a clip-like device called a probe is placed on a patient’s finger or ear lobe. The oximeter works by using an electronic processor and a pair of small light-emitting diodes (LEDs) facing a photodiode, which is why it is important that the device is placed on a translucent part of the patient’s body. The absorption of different wavelengths of light differs significantly in blood that is loaded with oxygen and blood that is lacking oxygen, with oxygenated hemoglobin (the main oxygen-carrying protein in blood) allowing more red light to pass through, and deoxygenated hemoglobin absorbing red light.
Pulse oximeters were widely used during the Covid-19 pandemic to monitor blood oxygen levels in affected patients, but at the panel committee meeting, researchers shared some concerning information: flawed readings on these devices may have contributed to the disproportionate number of deaths among Black and dark-skinned individuals.
The unfortunate reality is that pulse oximetry’s inability to accurately monitor patients with darker skin is not new to the medical community. Decades before the pandemic, studies showed that pulse oximeters would often give a healthy reading, even when blood tests showed more concerning blood saturation levels. It was found that melanin in skin can interfere with the absorption of light that the devices use, and at times, this can mask possibly dangerously low oxygen levels. Historically, pulse oximeters have been developed, tested, and calibrated largely on patients with lighter skin tones. In fact, FDA guidance for approving pulse oximeters states that clinical trials should include at least two darkly pigmented people, or 15% of the subject pool (whichever is larger). Experts have argued that this benchmark is insufficient, especially when taking into account the wide range of skin tones. Additionally, officials at the FDA panel hearing reported that pulse oximeters that were used in hospitals were marketed based on studies of as little as 10 healthy people. Even worse yet, many pulse oximeters that people buy online or from retail stores to monitor oxygen levels at home are not regulated at all by the FDA.
After hearing from researchers and physicians this week, the Anesthesiology and Respiratory Therapy Devices advisory panel urged the FDA to provide prominent warnings in retail devices that are for medical use and are not approved by the agency. Additionally, the panel has suggested that the FDA require more rigorous testing of the devices to address their racial biases.
Many experts have attributed the pulse oximeter’s shortcomings to the FDA’s failure to properly regulate these devices, a negligence in the medical community, and a lack of diversity in engineering spaces where new technology is created. And while all of these factors have likely contributed, one way or another, to the issues that we see today, one thing is clear: the consequences of such inaccuracies have been devastating. It is incredibly disheartening to know that a several-fold greater number of deaths during the Covid-19 pandemic in ethnic minority patients compared to white patients is what it took to drive the FDA towards any sort of action.